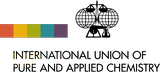Recent news
Selected CIAAW news are listed here in chronological order.
20 Dec 2024
Natural variations of carbon isotope ratios are expressed relative to Vienna Peedee belemnite (VPDB). In 1998, IUPAC recommended the value of the 13C/12C isotope ratio in VPDB as reported by Chang and Li (Chin. Sci. Bull. 35, 290-296), and this recommendation was further reaffirmed by IUPAC in 2010. However, recent measurements of carbon isotope ratios associated with VPDB have led the CIAAW to reexamine the set of reference values describing the isotopic composition of VPDB. The CIAAW now recommends the following value of the 13C/12C isotope ratio for VPDB (where the quoted uncertainty is at 95 % confidence level):
R(13C/12C, VPDB) = 0.011 113 ± 0.000 022
This value represents a consensus estimate from ten studies published between 1990 and 2024 from numerous independent measurement techniques. One of these studies is the work of Dunn et al. (Rapid Comm. Mass Spectrom. 38, e9773), which the CIAAW has identified as the best mass spectrometric measurement of the isotopic composition of carbon from a single terrestrial sample published in the peer-reviewed literature, commonly known as the “IUPAC Best Measurement.”
This revision also affects other quantities surrounding VPDB. Values shown here, along with their associated expanded uncertainties (at 95 % confidence level), are meant to replace those recommended by IUPAC in 2010.
δVPDB(13C/12C, NBS 19) = 0.001 95 (assumed exact)
δVPDB(18O/16O, NBS 19) = –0.0022 (assumed exact)
R(18O/16O, VPDB-CO2)/R(18O/16O, VPDB) = 1.010 25 *
R(18O/16O, VPDB)/R(18O/16O, VSMOW) = 1.030 92 *
λ = 0.528 *
R(17O/16O, VPDB-CO2) = 0.000 3907 ± 0.000 0012 **
R(18O/16O, VPDB-CO2) = 0.002 088 39 ± 0.000 000 92
R(17O/16O, VPDB-CO2)/R(13C/12C, VPDB) = 0.035 16 ± 0.000 09
Asterisk identifies commonly accepted values that are assumed exact. A datafile describing the provenance of these newly recommended values is available for download at ciaaw.org/data/vpdb-2024.xlsx.
These changes and considerations will be published in Chemistry International and later in Pure and Applied Chemistry.
(** The original Press Release had a value 0.000 3908 by mistake)
23 Oct 2024
The IUPAC Commission on Isotopic Abundances and Atomic Weights (CIAAW) met in August 2023 in the Hague, the Netherlands, under the leadership of Prof. Johanna Irrgeher (Montanuniversität Leoben, Austria).
Following this meeting, the Commission recommends changes to the standard atomic weights of gadolinium, lutetium, and zirconium based on recent determinations and evaluations of their terrestrial isotopic abundances.
The CIAAW notes that the standard atomic weight of gadolinium was last revised in 1969 based on isotopic abundance measurements made in the 1940s. Since then, several studies dedicated to the
measurement of the isotopic composition of gadolinium have been published which warrant a revised standard atomic weight. For lutetium and zirconium, there are more recent measurements available and their standard atomic weights were
last revised by IUPAC in 2007 and 1983, respectively. [See the full IUPAC Press Release]
23 Aug 2022
It is with great sadness that we announce that Norman E. Holden passed away on 18 August 2022, at the age of 86.
Norman was the 9th Chair of the CIAAW from 1980-1983, prior to which he also served as the Secretary from 1976-1979, and was the co-founder of what is now known as the IUPAC Subcommittee for Isotopic Abundance Measurements (SIAM).
He remained active member of the CIAAW and attended his last CIAAW meeting virtually in 2021.
6 May 2021
Following the publication of the IUPAC Technical Report on the variation of lead isotopic composition and atomic weight in terrestrial materials, the CIAAW has recommended changes to the standard atomic weight of lead.
The standard atomic weights of six other elements (fluorine, holmium, scandium, terbium, thulium, and yttrium),
all having a single stable isotope, have been revised based on the new assessment of their atomic masses endorsed by the International Union of Pure and Applied Physics.
These changes and considerations will be published in Chemistry International and later in Pure and Applied Chemistry. [See the full IUPAC Press Release for lead]
11 Dec 2019
The IUPAC Commission on Isotopic Abundances and Atomic Weights (CIAAW) met under the chairmanship of Dr. Juris Meija, at the Federal Institute for Materials Research and Testing, Berlin Germany, in June 2019.
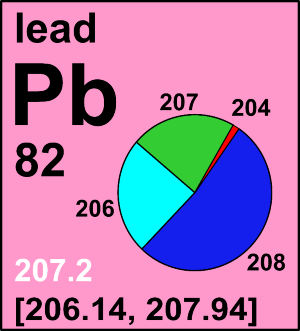
Following its 2019 meeting, the Commission recommended changes to the standard atomic weight of hafnium based on recent determinations and evaluations of its isotopic composition.
The CIAAW continues to evaluate literature data which leads to identification of developments in the measurement science, recognition of new discoveries, and remains committed to modernize its technical guidelines and work towards further expansion of its website to include more historical databases.
These changes and considerations will be published in Chemistry International and later in Pure and Applied Chemistry. [See the full IUPAC Press Release]
16 Nov 2018
The General Conference on Weights and Measures has voted unanimously in favor of the revised definitions of the kilogram, mole, ampere, and kelvin at its 26th meeting. These changes will take force on 20 May 2019.
While this revision of the International System of Units has no effect on the atomic weights of the elements, calculation of molar masses to a very high-precision will be affected by this change. Most notably, the molar mass of unbound carbon-12 atoms will no longer be 12 g/mol exactly. The new definition of the mole has been endorsed by IUPAC and can be consulted here.
5 Jun 2018
The IUPAC Commission on Isotopic Abundances and Atomic Weights (CIAAW) met under the chairmanship of Dr. Juris Meija, at the University of Groningen, the Netherlands, in September 2017.
Following its meeting, the Commission recommended changes to the standard atomic weights of 14 chemical elements.
The standard atomic weights of argon and iridium have been changed based on recent determinations and evaluations of terrestrial isotopic abundances.
For argon, assignment of an interval for the new standard atomic weight reflects the common occurrence of variations in the atomic weights in normal terrestrial materials [See the relevant IUPAC Technical Report].
The standard atomic weights of the other 12 elements (aluminium, cobalt, gold, holmium, manganese, niobium, praseodymium, protactinium, rhodium, terbium, thulium, and yttrium),
all having a single stable isotope, have been revised based on the new assessment of their atomic masses endorsed by the International Union of Pure and Applied Physics.
The CIAAW also notes that the international isotopic reference material LSVEC lithium carbonate which (together with NBS 19) is used to realize the carbon isotope delta scale (VPDB), is able to absorb carbon dioxide from air.
This process changes the carbon isotope ratio in LSVEC with time. Given that LSVEC is unsuitable as a reference material for carbon isotope ratio analysis, its use is no longer recommended.
Carbon isotope delta measurements must still be normalized to the VPDB scale using at least two suitable international reference materials selected by the users as appropriate.
These changes and considerations will be published in Pure and Applied Chemistry. [See the full IUPAC Press Release]
20 Dec 2017
The United Nations General Assembly has proclaimed 2019 the International Year of the Periodic Table of Chemical Elements [Resolution A/RES/72/228, Draft Resolution A/72/422/Add.2] to enhance global awareness of, and to increase education in, the basic sciences, with special attention to the countries of the developing world.
The year 2019 also marks the centenary of IUPAC which will provide an unique opportunity for IUPAC and all its members to highlight the importance of chemistry in an international enterprise. [See IUPAC Press Release].
28 Nov 2016
IUPAC Bureau has approved the official names and symbols for the elements 113 (nihonium, Nh), 115 (moscovium, Mc), 117 (tennessine, Ts), and 118 (oganesson, Og) [see the IUPAC Press Release].
The names of these elements follow the new 2016 IUPAC Rules for naming of new elements.
16 Aug 2016
As a result of IUPAC Project 2014-024-1-200,
new interactive electronic version of the IUPAC Periodic Table of the Elements and Isotopes has been launched. The IUPAC Interactive Electronic Periodic Table and accompanying resources can be accessed at www.isotopesmatter.com.
24 Apr 2016
IUPAC kindly welcomes comments on its Provisional Recommendation "Guidelines for the use of atomic weights" by 31 Aug 2016.
Please consult IUPAC website for more details (here) and direct your comments to Task Group Chair Adriaan M. H. van der Veen (avdveen@vsl).
18 Apr 2016
It is with great sadness that we announce that Paul De Bièvre passed away on 14 April 2016, at the age of 82.
Paul was an active member of CIAAW from 1971 and was the co-founder and the inaugural Chairman of what is now known as the IUPAC Subcommittee for Isotopic Abundance Measurements (SIAM).
His contributions to CIAAW, passion for the highest quality measurements and accuracy in communication will be lasting memories.
24 Feb 2016
CIAAW has published the "Atomic weights of the elements 2013 (IUPAC Technical Report)" which is dedicated to its 7th Chairman, Prof. Norman Neill Greenwood (1925-2012).
The report is freely available from IUPAC publisher DeGruyter here.
20 Feb 2016
CIAAW has revised the Table of Isotopic Compositions of the Elements (TICE). The update involved a critical evaluation of the recent published literature.
The new TICE 2013 includes evaluated data from the "best measurement" of the isotopic abundances in a single sample, along with a set of representative isotopic abundances and uncertainties that accommodate known variations in normal terrestrial materials.
The report is freely available from IUPAC publisher DeGruyter here.
24 Aug 2015
The IUPAC Commission on Isotopic Abundances and Atomic Weights met under the chairmanship of Dr. Juris Meija, at the University of Natural Resources and Life Sciences Vienna, Austria, prior to the 48th IUPAC General Assembly in Busan, Korea, in August 2015.
Following its meeting, the Commission recommended a change to the standard atomic weight of ytterbium. The IUPAC Bureau, at its meeting on 14 August, approved this change.
[See the full IUPAC Press Release]
CIAAW discussed the uncertainty interpretation of standard atomic weights and resolved to endorse probabilistic interpretation of standard atomic weights using various probability density functions, including the uniform probability distribution as outlined in the Eurachem/CITAC Guide when no specific knowledge is available about the isotopic composition of a material of interest.
In all cases, probability distributions describe the lack of knowledge about isotopic compositions of particular materials or classes of materials, and not the actual distribution of natural abundances.
25 Mar 2015
In Session I of the 104th meeting of the International Committee on Weights and Measures (CIPM), the CIPM adopted the traceability exception related to delta value isotope ratio measurements.
The CIPM decided (Decision CIPM/104-26) that a list of certified reference materials that should be used to identify accepted references for delta value isotope ratio traceability statements is published and maintained by IUPAC-CIAAW.
4 Mar 2014
CIAAW has published the "Assessment of international reference materials for isotope-ratio analysis (IUPAC Technical Report)".
The report is available from IUPAC publisher DeGruyter here.
24 Sep 2013
The IUPAC Commission on Isotopic Abundances and Atomic Weights met under the chairmanship of Dr. Willi A. Brand, at the Scientific and Technological Research Council of Turkey in Gebze, prior to the IUPAC General Assembly in Istanbul, Turkey, in August 2013.
Following its meeting, the Commission recommended changes to the standard atomic weights of 19 chemical elements. The IUPAC Council, at its meeting on 14-15 August, approved these changes. The changes are the result of cooperative research supported by the U.S. Geological Survey, IUPAC, and other contributing Commission members and institutions.
The standard atomic weights of cadmium, molybdenum, selenium, and thorium have been changed based on recent determinations of terrestrial isotopic abundances. In addition, the standard atomic weights of 15 elements have been revised based on the new assessment of their atomic masses by International Union of Pure and Applied Physics.
CIAAW has also resolved to reclassify thorium from a mono- to a bi-isotopic element owing to the significant abundance of the thorium-230 isotope in deep seawaters.
CIAAW also recommended a new standard value for the isotope ratio of uranium, N(238U)/N(235U) = 137.8(1), in naturally occurring terrestrial materials.
[See the full IUPAC Press Release]

CIAAW
The CIAAW recommends a revised value of the 13C/12C isotope ratio for Vienna Peedee belemnite (VPDB).

CIAAW
The standard atomic weight of gadolinium, lutetium, and zirconium have been changed based on recent evaluation of its isotopic composition (Oct 2024).

CIAAW
Norman Edward Holden (1936-2022)
Norman E. Holden served as the 9th Chair of the CIAAW from 1980-1983, prior to which he also served as the CIAAW Secretary from 1976-1979. In 2017 Norman was named an Honorary Member of the CIAAW.
CIAAW
The standard atomic weight of lead has been changed based on recent evaluation of its isotopic composition.
See the relevant IUPAC Technical Report by X.-K. Zhu et al.
CIAAW
The standard atomic weight of hafnium has been changed based on recent determinations and evaluations of its isotopic composition.
BIPM
New definitions for the kilogram, mole, ampere, and kelvin have been formally adopted by the 26th General Conference on Weights and Measures in Versailles, France on 16 November 2018.

CIAAW
The standard atomic weight of argon has been changed based on recent evaluation of terrestrial isotopic abundances. The assignment of an interval for the new standard atomic weight reflects the common occurrence of variations in the atomic weights in normal terrestrial materials.
See the relevant IUPAC Technical Report by J.K. Böhlke.

WIKIPEDIA COMMONS
The year 2019 marks the 150 anniversary of the Periodic Law which was developed independently by Dmitri Mendeleev and Lothar Meyer in 1869.
The United Nations has proclaimed 2019 the International Year of the Periodic Table of Chemical Elements.
CIAAW
In November 2016, IUPAC has officially adopted the names nihonium (Nh), moscovium (Mc), tennessine (Ts), and oganesson (Og) for elements 113, 115, 117, and 118, respectively.

M.Wieser/CIAAW
Paul De Bièvre (1933-2016)
Paul De Bièvre was born in Blankenberge (Belgium) on 7 July 1933 and he passed away on 14 April 2016 in Leuven (Belgium).
CIAAW
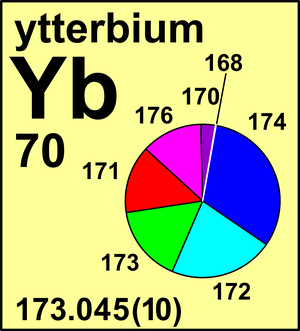
Ytterbium
For ytterbium, which was first obtained in a pure state just some 50 years ago, only two calibrated measurements of its isotopic composition have been ever made.
Both of these measurements differ from one another. Ytterbium exemplifies the situation that is also true for many other elements: well-documented isotope ratio measurements are still needed.