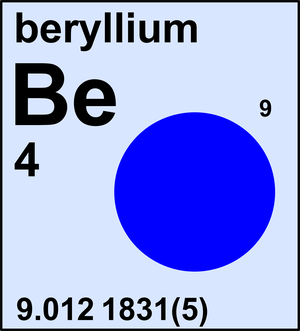
Beryllium
| Isotope | Atomic mass (Da) | Isotopic abundance (amount fraction) |
|---|---|---|
| 9Be | 9.012 1831(5) | 1 |
Beryllium is a monoisotopic element and its atomic weight is determined solely by its isotope 9Be. The Commission last revised the standard atomic weight of beryllium in 2013 based on the latest Atomic Mass Evaluation by IUPAP.
Before 1961, the atomic weight of beryllium was based on chemical determinations. In 1961, the Commission proposed Ar(Be) = 9.0122 based on atomic mass data and this value has been periodically refined to reflect the advances in nuclide mass measurements. In 2013, the Commission recommended Ar(Be) = 9.012 1831(5).
10Be is a cosmic-ray spallation product from N, O, Ne, and Ar and decays with a half-life of 1.6(2) Ma. 10Be is pervasive on the earth's surface in equilibrium abundances of less than 10-11, which is too small to affect the atomic weight of Be in normal materials. Beryllium is the only monoisotopic element with an even atomic number, but it has an odd mass number like all other monoisotopic elements.
© IUPAC 2003
CIAAW
Beryllium
Ar(Be) = 9.012 1831(5) since 2013
The name derives from the Greek word beryllos for "beryl", a gemstone in which
it is found (3BeO×Al2O3×6SiO2).
Beryllium was discovered by the French chemist and pharmacist Nicholas-Louis Vauquelin in beryl
and emerald in 1797. The element was first separated in 1828 by the French chemist Antoine-Alexandre-Brutus Bussy and independently by the German chemist Friedrich Wöhler. Because the salts
of beryllium have a sweet taste, the element was also known as glucinium from the Greek glykys for "sweet", until IUPAC selected the name beryllium in 1949.