Cadmium
| Isotope | Atomic mass (Da) | Isotopic abundance (amount fraction) |
|---|---|---|
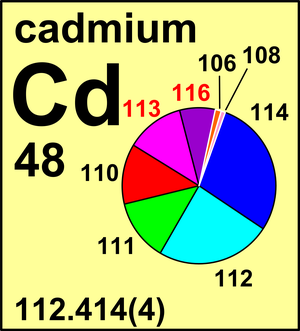
| 106Cd | 105.906 460(8) | 0.012 45(22) |
| 108Cd | 107.904 184(8) | 0.008 88(11) |
| 110Cd | 109.903 008(3) | 0.124 70(61) |
| 111Cd | 110.904 184(3) | 0.127 95(12) |
| 112Cd | 111.902 764(2) | 0.241 09(7) |
| 113Cd | 112.904 408(2) | 0.122 27(7) |
| 114Cd | 113.903 365(2) | 0.287 54(81) |
| 116Cd | 115.904 763(1) | 0.075 12(54) |
By 1961, the chemical measurements of Ar(Cd), seemingly consistent with each other, had averaged to 112.40 with little spread, whereas the mass-spectrometric measurements yielded 112.41 to 112.43 with the recommended atomic weight Ar(Cd) = 112.41(1).
In 1985, the Commission re-examined the available mass-spectrometric data and recommended a value of Ar(Cd) = 112.411(8), which was revised in 2013 to 112.414(4).
The "g" annotation arises from the presence of naturally occurring Cd fission products found in fossil reactors at Gabon, south-west Africa. 113Cd is β–, but its half-life is so long (1016 a) that it does not affect Ar(Cd) measurably even over geologic time periods. It decays into the minor isotope of In, but abnormal occurrences of that element with anomalous Ar(In) from the decay of 113Cd have not been reported.
© IUPAC 2003
CIAAW
Cadmium
Ar(Cd) = 112.414(4) since 2013
The name derives from Greek kadmeia for "calamine" (zinc carbonate), with which it was found as an
impurity in nature. It may have been found in furnace flue dust in Thebes, a city in the Boeottia region
of central Greece. The mythological king of Phoenicia, Cadmus, founded Thebes and would be a source
for the name of the ore. The element was discovered and first isolated by German physician Friedrich
Stromeyer in 1817.
Isotopic reference materials of cadmium.