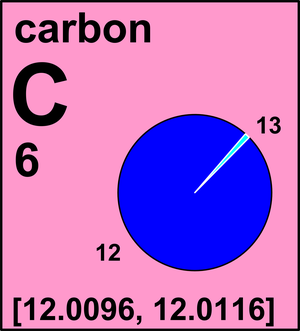
Carbon
| Isotope | Atomic mass (Da) | Isotopic abundance (amount fraction) |
|---|---|---|
| 12C | 12(exact) | [0.9884, 0.9904] |
| 13C | 13.003 354 835(2) | [0.0096, 0.0116] |
The 12C isotope has served since 1960 as the scale-determining reference for the definition of the unified atomic mass unit and is the basis of all atomic weights. The zero value for the delta scale used in relative isotope-ratio measurements of carbon since the 1950s was based on a sample of fossil marine carbonate (Belemnitella Americana, Peedee Formation, Cretaceous Period, South Carolina, also known as PDB).
In 1961, the Commission recommended Ar(C) = 12.011 15(5) and in 1969 it recommended Ar(C) = 12.011(1). The larger uncertainty was assigned to include all terrestrial sources of carbon whose isotopic compositions had been measured to that time. After the supply of PDB was exhausted, a modified delta scale was recommended for relative carbon isotope-ratio measurements (referred to as the Vienna PDB, or VPDB scale) that yields the same zero value as the PDB scale when based on measurements of a new carbonate reference material known as NBS 19. In 1995, the Commission recommended Ar(C) = 12.0107(8) as a result of a re-evaluation of variations in normal terrestrial materials.
Variations in the n(13C)/n(12C) ratio of terrestrial sources of carbon are caused largely by biogeochemical reactions and physical processes. Some of the largest effects are associated with oxidation-reduction reactions including photosynthesis, such that organic substances and reduced natural gases typically are depleted in 13C relative to carbonate materials and the atmosphere. Differences in the degree of 13C depletion during photosynthesis are characteristic of some groups of plants and may be passed along to plant consumers, such that carbon isotope studies can be used to identify features of animal diets and paleoclimates. Variations in the relative rates of organic carbon production, burial, and oxidation through geologic time are recorded in the isotopic compositions of sedimentary rocks. The highest reported 13C abundance is from dissolved carbonate in reduced marine sediment pore water with x(13C) = 0.011 466 and Ar(C) = 12.011 50. The lowest reported 13C abundance is from crocetane recovered from the ocean bottom at cold seeps in the northern Pacific Ocean with x(13C) = 0.009 629 and Ar(C) = 12.009 66.
The radioactive 14C isotope has a half-life of 5730 a. It is introduced continuously to the near-surface environment of the earth by cosmic-ray reactions, from cosmic dust, and by nuclear technology. It is of great interest for prehistoric dating as well as archaeological, anthropological, paleotemperature, and zoological studies. Yet, this isotope never occurs in normal carbon sources in concentrations high enough to affect significantly the Ar(C) value. Before nuclear weapons tests, the abundance of 14C in the atmosphere had an average value of only about 10−16. It should be noted that a half-life of 5568 a (the so-called "Libby half-life"), has been adopted by convention for calculations in geochronology.
Atomic weights of the elements 2009 by M.E. Wieser and T.B. Coplen. Pure Appl. Chem. 2011 (83) 359-396
CIAAW
Carbon
Ar(C) = [12.0096, 12.0116] since 2009
The name derives from the Latin carbo for "charcoal". It was known in prehistoric times in the form of
charcoal and soot. In 1797, the English chemist Smithson Tennant proved that diamond is pure carbon.
Natural variations of carbon isotopic composition
Isotopic reference materials of carbon.