Copper
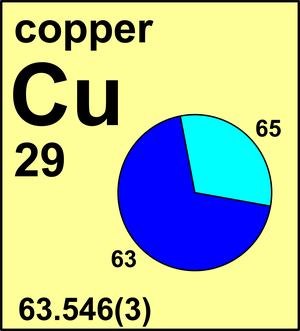
| Isotope | Atomic mass (Da) | Isotopic abundance (amount fraction) |
|---|---|---|
| 63Cu | 62.929 597(3) | 0.6915(15) |
| 65Cu | 64.927 790(5) | 0.3085(15) |
In 1961, the Commission recommended Ar(Cu) = 63.54 based on the recent chemical determinations. In 1967, the Commission recommended Ar(Cu) = 63.546(1) based on the mass-spectrometric measurements. The recommended uncertainty was increased to 0.003 in 1969 to include natural variations of up to approx. 0.15 % in the isotopic abundances of copper isotopes, and given the annotation "r" to indicate that the precision was limited by natural variability.
In the compilation by the Commission, the lowest reported δ65Cu value in a naturally occurring sample is −7.65 ‰ (x(65Cu) = 0.3066 and Ar(Cu) = 63.542) for a specimen of a Cu-chloride mineral (atacamite) from Chile. The highest reported δ65Cu is +9 ‰ (x(65Cu) = 0.3102 and Ar(Cu) = 63.549) for a Cu-carbonate mineral (aurichalcite) from Arizona. Some of these values are outside the range of the stated atomic-weight uncertainty and may justify a re-evaluation by the Commission of the atomic-weight uncertainty or annotations
© IUPAC 2003
CIAAW
Copper
Ar(Cu) = 63.546(3) since 1969
The name derives from the Latin cuprum for Cyprus, the island where the Romans first obtained copper.
The symbol Cu also comes from the Latin cuprum. The element has been known since prehistoric times.
Isotopic reference materials of copper.