Dysprosium
| Isotope | Atomic mass (Da) | Isotopic abundance (amount fraction) |
|---|---|---|
| 156Dy | 155.924 284(8) | 0.000 56(3) |
| 158Dy | 157.924 41(2) | 0.000 95(3) |
| 160Dy | 159.925 203(5) | 0.023 29(18) |
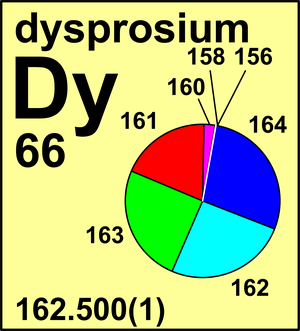
| 161Dy | 160.926 939(5) | 0.188 89(42) |
| 162Dy | 161.926 804(5) | 0.254 75(36) |
| 163Dy | 162.928 737(5) | 0.248 96(42) |
| 164Dy | 163.929 181(5) | 0.282 60(54) |
In 1969, the Commission assessed Ar(Dy) = 162.50(3). The atomic weight and uncertainty of dysprosium were changed to their current values in 2001 as a result of new mass-spectrometric measurements. The "g" notation arises from the presence of naturally occurring fission products found in fossil reactors at Gabon, south-west Africa.
© IUPAC 2003
CIAAW
Dysprosium
Ar(Dy) = 162.500(1) since 2001
The name derives from the Greek dysprositos for "hard to get at", owing to the difficulty in separating
this rare earth element from a holmium mineral in which it was found. It was discovered by the Swiss
chemist Marc Delafontaine in the mineral samarskite in 1878 and called philippia. Philippia was subsequently
thought to be a mixture of terbium and yttrium. It was later rediscovered in a holmium sample
by the French chemist Paul-Emile Lecoq de Boisbaudran in 1886, who was then credited with the
discovery. Dysprosium was first isolated by the French chemist Georges Urbain in 1906.