Helium
| Isotope | Atomic mass (Da) | Isotopic abundance (amount fraction) |
|---|---|---|
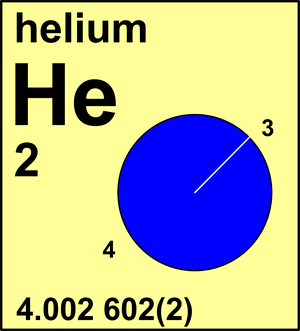
| 3He | 3.016 029 322(2) | 0.000 002(2) |
| 4He | 4.002 603 2545(4) | 0.999 998(2) |
In contrast to the other noble gases, which are separated for commercial use almost entirely from air, a large amount of commercial He is derived from natural gas deposits containing relatively high concentrations of radiogenic 4He. In its 1961 report, the Commission recommended Ar(He) = 4.0026 based on the atomic mass of 4He to four decimal places. The abundance of 3He in air of 0.000 137 % as determined by Nier had a negligible effect on the atomic weight of helium. This isotope is present in natural sources of helium with a smaller abundance than that of any other stable isotope relative to its elemental composition. In 1969 the Commission recommended Ar(He) = 4.002 60(1), which was identical to the atomic mass of 4He to six significant figures. A subsequent determination of the isotopic composition of atmospheric helium have confirmed this value which has been last revised in 1983.
The indicated interval for the standard atomic weight of He has a lower limit of 4.002 600, corresponding to an abundance of x(3He) = 3.3×10-6. This interval includes some natural 3He-enriched sources, but it does not include all helium from volcanic rocks or associated geothermal springs and gases, some of which have 3He abundances more than ten times that of atmospheric helium, hence the annotation "g". Those types of 3He-enriched sources are considered to represent emissions of primordial helium from incompletely degassed regions deep within the earth.
Helium, a noble gas, is chemically the most unreactive element with the lowest boiling point (4.2 K). Apart from its presence in gaseous or fluid inclusions and interstitial positions in crystals, and in voids in clathrate compounds, He occurs naturally only as a monoatomic gas. Helium is too light to be held in the atmosphere by Earth's gravitation over periods comparable with the age of the earth; thus, 4He in the atmosphere has been derived almost entirely from degassing of the solid earth, where it is produced by alpha decay of heavy radioisotopes. The minor isotope 3He is believed to be largely primordial, with minor amounts derived from beta decay of 3H, which is produced by the reaction 6Li(n,α)3H in the earth, by cosmic-ray reactions in the atmosphere, or by nuclear explosions and industry. Accumulations of 3He produced by radioactive decay of anthropogenic 3H provide a useful tool for determining ages of groundwaters and surface waters, including the ocean. The physical properties of 3He in gas, liquid, and solid phases and of 3He and 4He mixtures have been the subjects of many experimental studies that have contributed decisively to our understanding of quantum physics and chemistry
© IUPAC 2003
CIAAW
Helium
Ar(He) = 4.002 602(2) since 1983
The name derives from the Greek helios for "sun". The element was discovered by spectroscopy during
a solar eclipse in the sun's chromosphere by the French astronomer Pierre-Jules-Cesar Janssen in
1868. It was independently discovered and named helium by the English astronomer Joseph Norman
Lockyer.
Helium was thought to be only a solar constituent until it was later found to be identical to the helium
in the uranium ore cleveite by the Scottish chemist William Ramsay in 1895. The Swedish chemists Per Theodore Cleve and
Nils Abraham Langet independently found helium in cleveite at about the same time.