Palladium
| Isotope | Atomic mass (Da) | Isotopic abundance (amount fraction) |
|---|---|---|
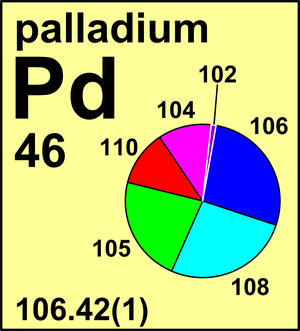
| 102Pd | 101.905 632(4) | 0.0102(1) |
| 104Pd | 103.904 030(9) | 0.1114(8) |
| 105Pd | 104.905 079(8) | 0.2233(8) |
| 106Pd | 105.903 480(8) | 0.2733(3) |
| 108Pd | 107.903 892(8) | 0.2646(9) |
| 110Pd | 109.905 173(5) | 0.1172(9) |
In 1969, the Commission recommended the standard atomic weight of Pd to be Ar(Pd) = 106.4(1) which gave palladium the least precisely tabulated atomic weight at that time. A decade later, using new mass-spectrometric measurements and evidence of lack of significant natural variations, the Commission recommended Ar = 106.42(1).
The "g" annotation arises from the presence of naturally occurring fission products found in fossil reactors at Gabon, south-west Africa.
© IUPAC 2003
CIAAW
Palladium
Ar(Pd) = 106.42(1) since 1979
The name derives from the second largest asteroid of the solar system Pallas (named after the goddess
of wisdom and arts—Pallas Athene). The element was discovered by the English chemist and physicist
William Hyde Wollaston in 1803, one year after the discovery of Pallas by the German astronomer
Wilhelm Olbers in 1802. The discovery was originally published anonymously by Wollaston to obtain
priority, while not disclosing any details about his preparation.