Rubidium
| Isotope | Atomic mass (Da) | Isotopic abundance (amount fraction) |
|---|---|---|
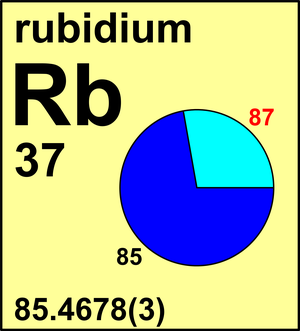
| 85Rb | 84.911 789 74(3) | 0.7217(2) |
| 87Rb | 86.909 180 53(4) | 0.2783(2) |
In its 1961 report, the Commission recommended Ar(Rb) = 85.47 based on the average of Ar(Rb) = 85.473 for the chemical determinations and mass-spectrometric determination of Nier, who reported Ar(Rb) = 85.4678(2). In 1969, the Commission recommended the current value of Ar(Rb) = 85.4678(3) based on new isotope-abundance measurements.
87Rb is β– active with a half-life of 48.8(5) Ga, which leaves Ar(Rb) unaffected at the currently given precision of about 3 parts per million in up to 106 a. In contrast, accumulation of the 87Sr product of 87Rb decay causes anomalous atomic-weight values of strontium in many Rb-bearing materials.
© IUPAC 2003
CIAAW
Rubidium
Ar(Rb) = 85.4678(3) since 1969
The name derives from the Latin rubidus for "deepest red" because of the two deep red lines in its spectra.
Rubidium was discovered in the mineral lepidolite by the German chemist Robert Wilhelm Bunsen and the
German physicist Gustav-Robert Kirchoff in 1861. Bunsen isolated rubidium in 1863.
Isotopic reference materials of rubidium.