Ruthenium
| Isotope | Atomic mass (Da) | Isotopic abundance (amount fraction) |
|---|---|---|
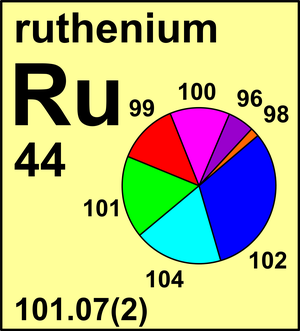
| 96Ru | 95.907 589(1) | 0.0554(14) |
| 98Ru | 97.905 29(5) | 0.0187(3) |
| 99Ru | 98.905 930(3) | 0.1276(14) |
| 100Ru | 99.904 211(3) | 0.1260(7) |
| 101Ru | 100.905 573(3) | 0.1706(2) |
| 102Ru | 101.904 340(3) | 0.3155(14) |
| 104Ru | 103.905 43(2) | 0.1862(27) |
In its 1961 report, the Commission changed the recommended atomic-weight value, Ar(Ru), from 101.1 to 101.07. In 1969, an uncertainty of 0.03 was assigned to that value. In view of the excellent agreement between the reported data, the Commission recommended in 1983 a reduction in the uncertainty of the standard atomic weight to Ar(Ru) = 101.07(2). The annotation "g" refers to anomalous occurrences at the Oklo natural nuclear reactor.
© IUPAC 2003
CIAAW
Ruthenium
Ar(Ru) = 101.07(2) since 1983
The name derives from the Latin ruthenia for the old name of Russia. It was discovered in a crude platinum
ore by the Russian chemist Gottfried Wilhelm Osann in 1828. Osann thought that he had found
three new metals in the sample, pluranium, ruthenium, and polinium. In 1844, Russian chemist Karl
Karlovich Klaus was able to show that Osann's mistake was due to the impurity of the sample, and
Klaus was able to isolate the ruthenium metal.