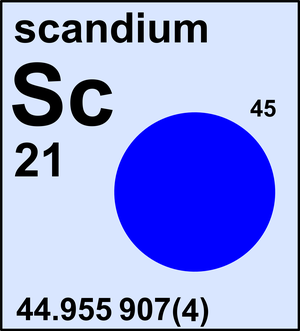
Scandium
| Isotope | Atomic mass (Da) | Isotopic abundance (amount fraction) |
|---|---|---|
| 45Sc | 44.955 907(4) | 1 |
Scandium is a monoisotopic element and its atomic weight is determined solely by its isotope 45Sc. The Commission last revised the standard atomic weight of scandium in 2021 based on the latest Atomic Mass Evaluation by IUPAP.
© IUPAC 2003
CIAAW
Scandium
Ar(Sc) = 44.955 907(4) since 2021
The name derives from the Latin scandia for Scandinavia, where the mineral was found. It was discovered
by the Swedish chemist Lars-Fredrik Nilson in 1879 in an ytterbium sample. In the same year,
the Swedish chemist Per Theodore Cleve proved that scandium was Mendeleev's predicted "eka-boron".