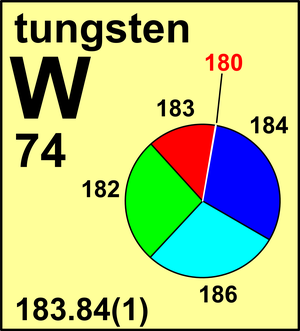
Tungsten
| Isotope | Atomic mass (Da) | Isotopic abundance (amount fraction) |
|---|---|---|
| 180W | 179.946 71(1) | 0.0012(1) |
| 182W | 181.948 206(5) | 0.2650(16) |
| 183W | 182.950 224(5) | 0.1431(4) |
| 184W | 183.950 933(5) | 0.3064(2) |
| 186W | 185.954 365(8) | 0.2843(19) |
In 1969, after evaluating the uncertainties associated with the mass-spectrometric measurements, the Commission assigned a value of Ar(W) = 183.85(3). For a number of years after that, in the absence of calibrated mass-spectrometric measurements, the Commission was concerned about a discrepancy between the recommended atomic weight and the results of earlier chemical determinations that yielded values of around Ar(W) = 183.90. In 1991, the Commission changed the recommended value for the atomic weight of tungsten to Ar(W) = 183.84(1), based on high-precision measurements with negative thermal ionization mass spectrometry.
© IUPAC 2003
CIAAW
Tungsten
Ar(W) = 183.84(1) since 1991
The name derives from the Swedish tungsten for "heavy stone". The symbol W derives from
the German wolfram, which was found with tin and interfered with the smelting of tin. It was said to
eat up tin like a wolf eats up sheep. The element was discovered by the Swedish pharmacist and chemist
Carl-Wilhelm Scheele in 1781. Tungsten metal was first isolated by the Spanish chemists Fausto
Elhuyar and his brother Juan José in 1783.