Xenon
| Isotope | Atomic mass (Da) | Isotopic abundance (amount fraction) |
|---|---|---|
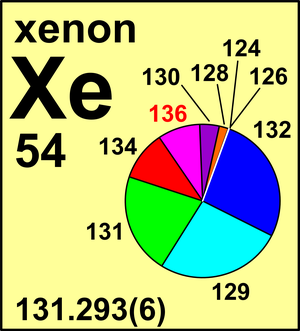
| 124Xe | 123.905 89(1) | 0.000 95(5) |
| 126Xe | 125.904 30(3) | 0.000 89(3) |
| 128Xe | 127.903 531(7) | 0.019 10(13) |
| 129Xe | 128.904 780 86(4) | 0.264 01(138) |
| 130Xe | 129.903 509 35(6) | 0.040 71(22) |
| 131Xe | 130.905 084 14(6) | 0.212 32(51) |
| 132Xe | 131.904 155 09(4) | 0.269 09(55) |
| 134Xe | 133.905 393 03(6) | 0.104 36(35) |
| 136Xe | 135.907 214 48(5) | 0.088 57(72) |
The standard atomic weight of xenon is based on analyses of xenon separated from air. In 1955, the Commission adopted a value of Ar(Xe) = 131.30, based on the isotope-abundance measurements of Nier. In 1961, the Commission recognized that this calculation was slightly in error and noted that with the same isotope abundances and using the most recent atomic masses, the correct calculated value was nearer to Ar(Xe) = 131.29. Despite this inconsistency, the Commission corrected this inconsistency and recommended Ar(Xe) = 131.29(3) only in 1979.
In 1999, in light of new mass-spectrometric measurements and a re-evaluation of previous data, the Commission recommended Ar(Xe) = 131.293(6), which remains to this day.
Xenon samples with relatively high 129Xe concentrations extracted from some types of primitive volcanic rocks and from some natural gas wells have been attributed to the decay of extinct 129I early in the Earth's history. Other reported minor occurrences of xenon of anomalous isotopic composition have been attributed to production of the heavy isotopes 131Xe to 136Xe from spontaneous and induced fission of uranium and fission of extinct 244Pu, production of 128Xe and 130Xe from double β– decay of 128Te and 130Te, and primordial sources. Not all of these variations are included within the atomic weight uncertainty, hence the annotation "g". Localized occurrences of isotopically anomalous Xe are associated with nuclear bomb test sites, and minor fractionations can occur during separation of Xe from air or other processes, hence the annotation "m".
© IUPAC 2003
CIAAW
Xenon
Ar(Xe) = 131.293(6) since 1999
The name derives from the Greek xenos for "the stranger". It was discovered by the Scottish chemist
William Ramsay and the English chemist Morris William Travers in 1898 in a liquefied air sample.