Ytterbium
| Isotope | Atomic mass (Da) | Isotopic abundance (amount fraction) |
|---|---|---|
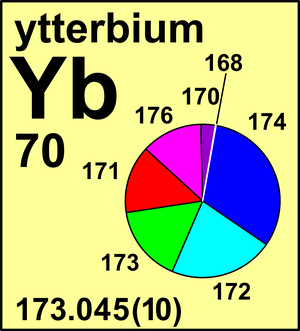
| 168Yb | 167.933 889(8) | 0.001 26(1) |
| 170Yb | 169.934 767 25(7) | 0.030 23(2) |
| 171Yb | 170.936 331 52(9) | 0.142 16(7) |
| 172Yb | 171.936 386 66(9) | 0.217 54(10) |
| 173Yb | 172.938 216 22(8) | 0.160 98(9) |
| 174Yb | 173.938 867 55(8) | 0.318 96(26) |
| 176Yb | 175.942 5747(1) | 0.128 87(30) |
The atomic weight of ytterbium has been taken as 173.04 since 1934. This chemically determined value
was reconfirmed by the Commission in 1961. However, from that time, Ar(Yb) was no longer based
on chemical but on mass-spectrometric data. In light of new mass-spectrometric determinations, the Commission recommended Ar(Yb) = 173.054(5) in 2007.
In 2015, the standard atomic weight of ytterbium was revised to 173.045(10). Ytterbium, which was first obtained in a pure state just some 50 years ago, only two calibrated measurements of its isotopic composition have been ever made.
Both of these measurements differ from one another and ytterbium exemplifies the situation that is also true for many other elements: well-documented isotope ratio measurements are still needed.
The "g" annotation is derived from anomalous Yb in samples from the Oklo uranium deposit in Gabon, south-west Africa.
CIAAW
Ytterbium
Ar(Yb) = 173.045(10) since 2015
The name derives from the Swedish village of Ytterby where the mineral ytterbite (the source of ytterbium)
was originally found. It was discovered by the Swiss chemist Jean-Charles Galissard de
Marignac in 1878 in erbium nitrate from gadolinite (ytterbite renamed).